Prof. In Su Kim’s Research Team Presented a Blueprint for the Pharmaceutical Synthesis Process
using the Reactivity of Cobalt Catalyst

▲ (from the left) Prof. In Su Kim, Dr. Prithwish Ghosh, Ph.D. Student Nayeon Kwon
Prof. In Su Kim’s Research Team (School of Pharmacy, first author: Dr. Prithwish Ghosh, co-author: Ph.D. student Nayeon Kwon) has identified for the first time in the world that cobalt catalysts can be used importantly in the structural transformation of heterocycles, the core skeleton of pharmaceuticals. In addition, it was revealed that the novel reactivity of the cobalt catalyst can be effectively applied to the synthesis process of high-cost drugs such as antiviral drugs, pulmonary fibrosis treatment drugs, and bronchodilators.
Cobalt is a transition metal element abundantly present on earth and has low cost and low human and environmental hazards. It is known as the central metal of vitamin B12, which must be ingested for human DNA biosynthesis, and is used as a key material necessary for controlling the risk of corrosion and explosion of the anode of lithium-ion batteries for electric vehicles.
Recently, initial studies have reported that cobalt catalysts may be involved in the production of carbon radicals. The previously reported carbon radical production method was possible under strong acid and photocatalytic conditions, but due to explosive, limited substrate application, and lack of location selectivity, application to actual pharmaceutical synthesis has been recognized as a limitation.
Through this study, Prof. Kim’s research team found that ‘Cobalt Ⅱ’, which is widely present in nature, is essential for the creation of carbon radicals by a new combination of a catalyst and potassium bromate, and it effectively acts on the location-selective combination of heterocycles, which are the core unit structures of pharmaceuticals, and carbon radicals. In addition, it was suggested that carbon radicals may be selectively introduced in drugs such as antiviral treatment nucleoside, pulmonary fibrosis treatment pirfenidone, and bronchodilator etofylline.
Prof. Kim explained the significance of the study by saying, “This research result is a new discovery that the use of cobalt catalyst and potassium bromate is very important for the formation of carbon radicals, especially regarding the stable utilization of carbon radicals. This study is an optimal synthesis method that can be effectively applied to design new drugs and shorten the manufacturing process of existing drugs by domestic and foreign pharmaceutical companies.”
This research was carried out with the support of the Basic Research Laboratory Support Project (BRL) and mid-level researcher support project promoted by the Ministry of Science and ICT and the National Research Foundation of Korea.
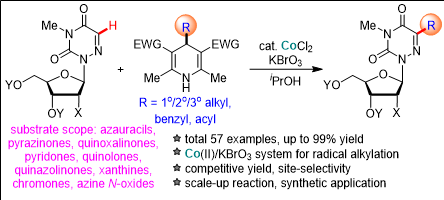
▲ Reaction formula in which ‘Cobalt Ⅱ’ converts the carbon-hydrogen bond in the antiviral nucleoside nucleobase into an alkyl group through a combination of catalyst and potassium bromate
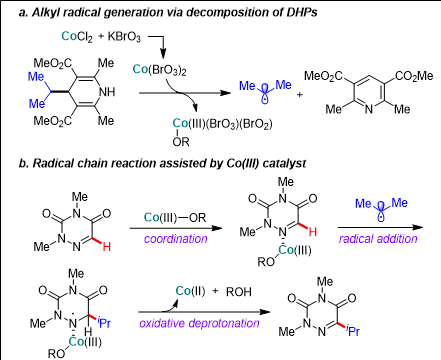
▲ The process in which the Hantzsch substrate, which can be used as an alkylation and acylation substrate, is decomposed by the cobalt catalyst and potassium bromate to form carbon radicals (figure a)
The process by which the generated carbon radical is selectively combined with carbon 5 in azauracil with the help of the cobalt Ⅲ catalyst, and then the desired product is formed through the oxidative dehydrogenation reaction of the cobalt trivalent catalyst (figure b).
- nucleoside analogues: Drugs that are structurally similar to DNA or RNA by modifying phosphate, sugar, and base, which are the basis of DNA and RNA
- heterocycles: Organic chemicals containing nitrogen or oxygen atoms in a ring as the core unit structure of medicines
- alkylation: A reaction that introduces an alkyl group into an organic compound through a substitution reaction or addition reaction
- acylation: A reaction that introduces an acyl or carbonyl group into an organic compound through a substitution reaction or addition reaction